
That costs a reasonable amount of energy. If methane were to dissolve, it would have to force its way between water molecules and so break hydrogen bonds. The problem is the hydrogen bonds between the water molecules. Methane is a gas, and so its molecules are already separate - the water doesn't need to pull them apart from one another. Why doesn't methane, CH 4, dissolve in water? Those which do dissolve often react with the water, or else are capable of forming hydrogen bonds with the water. Most molecular substances are insoluble (or only very sparingly soluble) in water.
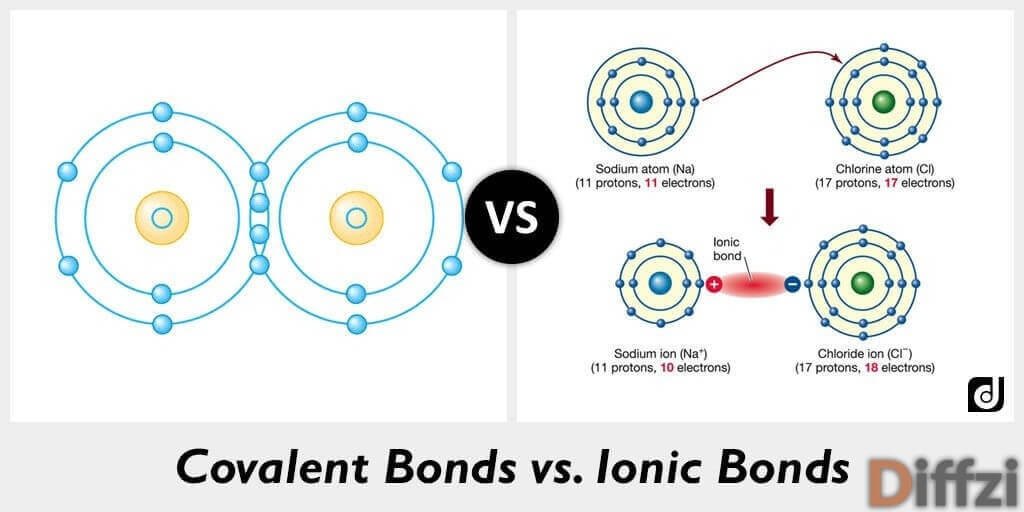
The larger the molecule the more van der Waals attractions are possible - and those will also need more energy to break. The presence of hydrogen bonding will lift the melting and boiling points. The size of the melting or boiling point will depend on the strength of the intermolecular forces. Exactly the same water molecules are present in ice, water and steam. Note: This is really important! You can make yourself look extremely stupid if you imply in an exam that boiling water, for example, splits it into hydrogen and oxygen by breaking covalent bonds. You don't have to break any covalent bonds in order to melt or boil a molecular substance. Molecular substances tend to be gases, liquids or low melting point solids, because the intermolecular forces of attraction are comparatively weak. Physical properties are governed by the intermolecular forces - forces attracting one molecule to its neighbours - van der Waals attractions or hydrogen bonds.
The covalent bonds holding the molecules together are very strong, but these are largely irrelevant to the physical properties of the substance. Molecules are made of fixed numbers of atoms joined together by covalent bonds, and can range from the very small (even down to single atoms, as in the noble gases) to the very large (as in polymers, proteins or even DNA). The physical properties of molecular substances Follow these links first if you aren't sure about these. Important! There's not much point in reading this page unless you are reasonably happy about the origin of hydrogen bonding and van der Waals forces. This page describes how the physical properties of substances having molecular structures varies with the type of intermolecular attractions - hydrogen bonding or van der Waals forces. Physical properties of molecular substances


 0 kommentar(er)
0 kommentar(er)
